Abstract
Introduction: Individualization of factor VIII (FVIII) prophylaxis dosing frequency is recommended in order to optimize treatment effectiveness in hemophilia A patients. While previous studies have shown long-term efficacy of BAY 81-8973, its long-term effectiveness in subgroups of patients receiving lower frequency prophylaxis (2x/week) has not been investigated. This post-hoc analysis assessed the association of BAY 81-8973 prophylaxis frequency with treatment adherence and long-term bleed outcomes in the LEOPOLD I extension study.
Methods: LEOPOLD I was a randomized, open-label, controlled, phase II/III cross-over trial of BAY 81-8973 routine prophylaxis for treatment of bleeds in adolescents and adults with severe hemophilia A. LEOPOLD I consisted of a 12-month main study period, followed by an optional 12-month extension period to assess longer-term safety and efficacy. In both study periods, patients received 20-50 IU/kg of BAY 81-8973 either 2x/week or 3x/week, with specific prophylaxis regimens determined by study investigators. Overall, 55 patients who completed the main study participated in the extension study period (2x/week: n = 17; 3x/week: n = 38).
This post-hoc analysis summarizes bleed outcomes and treatment adherence during the main and extension study periods among these 55 patients. Adherence was calculated over all recorded periods of BAY 81-8973 prophylaxis use in the main and extension periods. In particular, percentage adherence was calculated over a patient's entire period of prophylaxis use by dividing the total number of prophylaxis infusions recorded in the patient's infusion diary by the total number of prophylaxis infusions that would be expected over this period under the physician prescribed weekly frequency of 2x/week or 3x/week. Periods of time during which patients received non-prophylaxis infusions were not considered in the calculation of percentage adherence. Differences between groups were assessed using Wilcoxon rank sum tests for continuous variables, and chi-square or Fisher's exact tests for categorical variables.
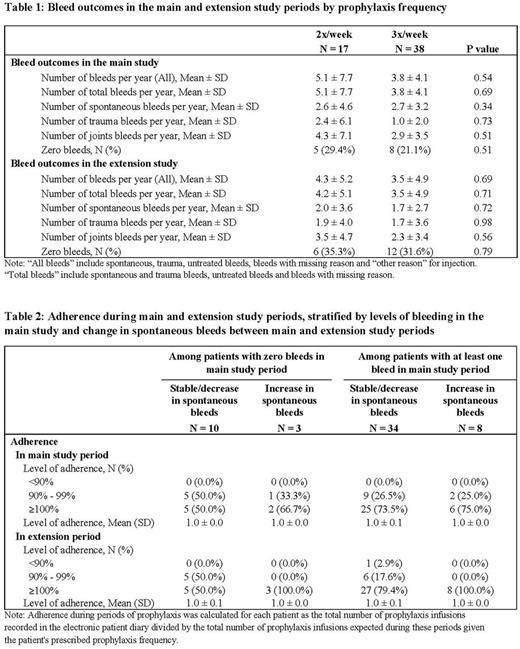
Results: Patients' mean age at the start of the main study was 31.5 (SD = 12.7).While the average number of bleeds was numerically slightly higher in the 2x/week group for most outcomes, there was no evidence of significant differences between 2x/week and 3x/week patients in either the main or extension study periods (Table 1). The proportion of patients with a stable or decreased number of spontaneous bleeds between the main and extension study periods was similar in the two groups (76.5% in 2x/week; 81.6% in 3x/week). Of the 13 patients (5 on 2x/week, 8 on 3x/week) who were bleed free after the main study period, 9 (3 on 2x/week, 6 on 3x/week) remained bleed free during the extension period as well. Adherence to prophylaxis infusions was very high in both the main (all 55 patients had ≥ 90% adherence) and extension study periods (54 of 55 patients were ≥ 90% adherent) (Table 2).
Conclusions: Bleed outcomes in the second year of follow-up were comparable between patients assigned by investigators to 2x/week versus 3x/week BAY 81-8973 prophylaxis. Bleed outcomes were stable or improved between the main and extension study periods in both groups, and a similar proportion of patients in both groups remained bleed free across both study periods. As nearly all patients were highly adherent, the absence of differences in bleed outcomes cannot be explained by greater adherence in lower frequency patients. As physical activity was not measured in this study, future studies should seek to rule out differences in activity that would impact bleed outcomes in patients receiving different prophylaxis treatment regimens. Overall, these results indicate that lower frequency prophylaxis is effective for long-term control of bleeding in selected patients who remain adherent to the BAY 81-8973 prophylaxis treatment regimen.
Saxena: Bayer HealthCare: Employment. Sajeev: Bayer HealthCare: Consultancy; Analysis Group, Inc.: Employment. Ayyagari: Bayer HealthCare: Consultancy; Analysis Group, Inc.: Employment. Dua: Bayer HealthCare: Consultancy; Analysis Group, Inc.: Employment. Church: Bayer HealthCare: Employment. Sanabria: Bayer HealthCare: Employment. Afonja: Bayer HealthCare: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal